Application
Hospital Acquired Infections
The rise of Multi Drug-Resistant Organisms has become a global health and economic concern, and tools to combat them are urgently needed.
Application
Gastrointestinal Infections
A successful forensic investigation begins with a proper sample, and proper samples are obtained using the appropriate collection method.

Application Brochure
Multi Drug-Resistant Organisms

Technology
Liquid-Based Microbiology™

Scientific study
Evaluation of Copan FecalSwab™ preserved stool specimens with the BD MAX™ Enteric Bacterial Panel and the BD MAX™ Extended Enteric Bacterial Panel
Hanen Fernandez Rojas
Scientific study
Evaluation of Copan FecalSwab as Specimen Type for Use in Xpert C. difficile Assay
Michael J. Mashock
Scientific study
Nonaplex PCR using Cliffhanger primers to identify diarrhoeagenic Escherichia coli from crude lysates of human faecal samples
Uffe Vest Schneider
Scientific study
Evaluation of Anatomically Designed Flocked Rectal Swabs for Use with the BioFire FilmArray Gastrointestinal Panel for Detection of Enteric Pathogens in Children Admitted to Hospital with Severe Gastroenteritis
Celia R. Walker
Scientific study
Evaluating the preservation and isolation of stool pathogens using the COPAN FecalSwab™ Transport System and Walk-Away Specimen Processor
Lee W Goneau
Scientific study
Rapid and accurate eXDR screening: use Xpert Carba-R® with FecalSwab®
Eric Farfour
Scientific study
Differences in Illness Severity among Circulating Norovirus Genotypes in a Large Pediatric Cohort with Acute Gastroenteritis
Sudha Bhavanam
Scientific study
Limited Genetic Diversity of blaCMY-2-Containing IncI1-pST12 Plasmids from Enterobacteriaceae of Human and Broiler Chicken Origin in The Netherlands
Evert P. M. den Drijver
Scientific study
Prospective Evaluation of the mariPOC Test for Detection of Clostridioides difficile Glutamate Dehydrogenase and Toxins A/B
Roosa Savolainen
Scientific study
High prevalence of multidrug resistant Enterobacteriaceae among residents of long term care facilities in Amsterdam, the Netherlands
Eline van Dulm
Scientific study
Prevalence of diarrheagenic Escherichia coli and impact on child health in Cap-Haitien,Haiti
Jenna N. Diaz et al
Scientific study
Gut microbiome profiling of neonates using Nanopore MinION and Illumina MiSeq sequencing
Teahyen Cha et al
Scientific study
Enhanced culture recovery of Campylobacter with modified Cary-Blair medium: A practical field experience
C Massip et al
Scientific study
Vertebral osteomyelitis caused by Campylobacter jejuni in an immunocompetent patient
Karina Frahm Kirk et al.
Scientific study
Detection of Enteropathogens in Human Immunodeficiency Virus and Non-Human ImmunodeficiencyVirus-Infected Children with Acute Diarrhea in an Indonesian Tertiary Hospital Using Multiplex Real-Time Polymerase Chain Reaction
Dewi Wulandari et al.
Fair
Medlab Middle East
For the expected, unexpected and everything in between
Medlab is the key event to unite Middle East healthcare and medical laboratory community, and is the right place to discover innovative products, trending technology, and build infinite networking opportunities. This year we’ll be there in person: discover our FLOQ technology – which revolutionized sample collection – and see first-hand our Liquid-Based Microbiology media, today the gold standard in microbiological sampling. Moreover, it will be a great chance to learn more about UniVerse®, our solution designed to bring efficiency, standardization, and safety in the service of thousands of molecular biology laboratories worldwide.
Fair
IBMS Congress
Delivering science, celebrating achievement
The COVID-19 pandemic has put science and scientists center stage in a way rarely seen. Being IBMS’s most important event for developing professional skills and knowledge, 2022’s Congress will truly celebrate science in all its variety, influence, potential, and impact on society. We, Copan people, are trembling to contribute to this event: we’ll be present there to display our patented technologies, our Liquid-Based Microbiology media, and lab automation. This year will be a great chance to learn more about UniVerse®, our solution designed to bring efficiency, standardization, and safety in the service of thousands of molecular biology laboratories worldwide. But that’s not all: in the dedicated executive suite 7 we’ll go deeper into sharing knowledge and communicating science: Stay tuned to discover what we arranged!
Fair
32nd ECCMID – European Congress of Clinical Microbiology & Infectious Diseases
The main event of the year! ECCMID brings together experts from many fields to present their latest findings, guidelines, and experiences to an audience of over 14,000 colleagues. The scientific program includes Keynote Lectures and Oral Sessions, Educational Workshops, Open Forums, Meet-The-Expert Sessions, and a wide range of Scientific Symposia. We are arranging great things for the occasion, including an Integrated Lunch Symposia – Saturday, April 23rd, from 12:15 to 13:15 – talking about self-collection experiences in different fields.
Fair
IBMS Congress 2023
Linking learning to the laboratory
The COVID-19 pandemic has put science and scientists center stage in a way rarely seen. Being IBMS’s most important event for developing professional skills and knowledge, 2022’s Congress will truly celebrate science in all its variety, influence, potential, and impact on society. We, Copan people, are trembling to contribute to this event: we’ll be present there to display our patented technologies, our Liquid-Based Microbiology media, and lab automation. This year will be a great chance to learn more about UniVerse®, our solution designed to bring efficiency, standardization, and safety in the service of thousands of molecular biology laboratories worldwide.
Microbiology time
Microbiology Time – March 2022
Spring is almost here, and together with sunny days and warm temperatures, we bring you new scientific studies for our March Microbiology time just uploaded on our database. This month’s best pick are two studies on Antibiotic-resistant Organisms and a review on the benefits of lab automation.

Microbiology Time
Microbiology Time – June 2023
This month we bring you two studies on the pediatric use of FecalSwab, and an outstanding paper illustrating the innovations introduced in forensic science by the French gendarmerie. Enjoy!

Microbiology Time
Microbiology Time – July 2023
For this July Microbiology Time, we selected three papers from Italian research teams. The first two explored the link between microbiome and cancer, while the third assessed bacterial water contamination in a dental clinic setting. Discover the full studies!

Microbiology Time
Microbiology Time – December 2023
In the last edition of this year’s Microbiology Time, three exciting topics: a novel bacterial strain in the vaginal microbiome, a case report of bacteria-induced osteomyelitis, and olfactory mucosa markers of frontotemporal dementia.

Microbiology Time
Microbiology Time – April 2024
In this ECCMID month, we selected three studies that span the microbiome and COVID-19 correlation to gastrointestinal diseases and tuberculosis. Read more in April’s Microbiology Time!

News
Copan FecalSwab® receives the 510(k) FDA clearance for molecular use with the BD MAX™ Enteric & Extended Enteric Bacterial Panels
Copan announces the 510(k) FDA clearance of its Copan FecalSwab® for collecting and transporting stool samples to be processed with BD Enteric Bacterial Panel and BD Extended Enteric Bacterial Panel on the BD MAX™ System. This clearance opens new molecular diagnostic opportunities for customers who already use FecalSwab® to maintain enteric pathogens’ vitality for microbial culturing.
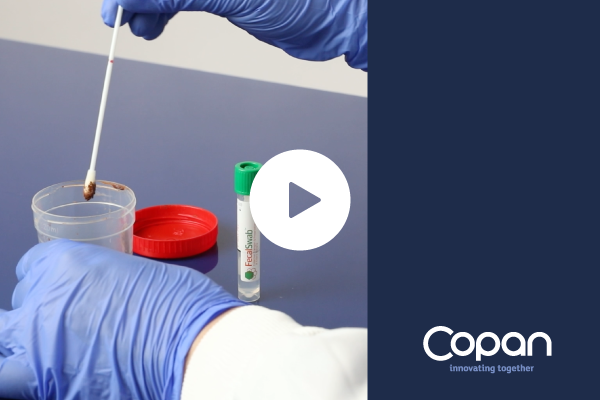
Video
FecalSwab® 4E048S – How to use
FecalSwab™ is intended to collect fecal specimens and preserve enteric pathogenic bacteria’s viability during transport to the testing laboratory. In the laboratory, FecalSwab™ samples are processed using standard clinical laboratory operating procedures for culture.
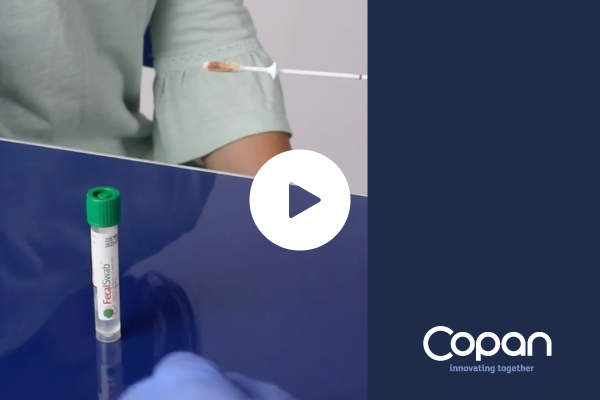
Video
FecalSwab® 470CE – How to use
FecalSwab™ is intended to collect fecal specimens and preserve enteric pathogenic bacteria’s viability during transport to the testing laboratory. In the laboratory, FecalSwab™ samples are processed using standard clinical laboratory operating procedures for culture.