Microbiology Time
It’s October Microbiology Time! Look at our database to find the latest studies taking advantage of our products. This month’s top picks are:
- An Italian study describing the presence of SARS-CoV-2 RNA in saliva samples from children attending nine schools in Rome. These results were analyzed in parallel with the trend of SARS-CoV-2 cases observed in the population of the same area, analyzed with nasopharyngeal swabs. Interestingly, the study found a different percentage of positivity in the two populations; nevertheless, the trend of SARS-CoV-2 RNA in saliva samples was consistent with that observed in the total population, suggesting that saliva is an excellent specimen to monitor SARS-CoV-2 diffusion in the population.
- A cost-benefit analysis for laboratories and criminal justice systems about the use of 4N6FLOQSwabs versus traditional cotton swabs: although the costs and benefits may vary locally and for different practices and policies, the overall outcome of this analysis highlights that the cost of using the 4N6FLOQSwabs’ more expensive technology pales compared with the potential tangible and intangible benefits.
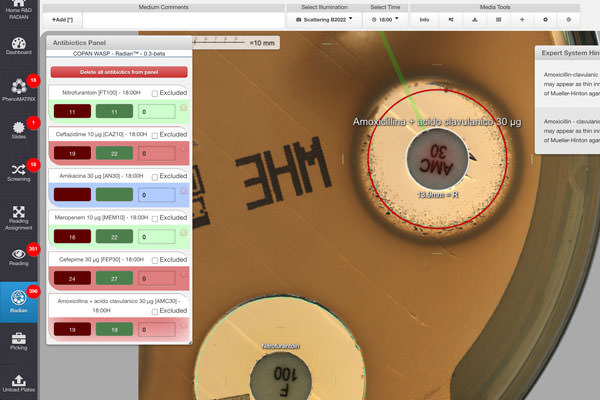
- A study by A. Chekraoui and colleagues that evaluates the accuracy and robustness of a fully automated Rapid Antimicrobial Susceptibility Testing directly from positive blood cultures (BCs). This study was conducted in two phases, the first with spiked BC bottles and a prospective clinical trial including more than 500 positive BC sequentially processed in routine at the Bacteriology Laboratory of Geneva University Hospitals. The RAST results were assessed against EUCAST standardized disk diffusion testing results. What did the study find? Despite some challenges with Pseudomonas aeruginosa and suboptimal performances with piperacillin-tazobactam, ceftazidime, and cefepime at the shortest time point (6 hrs) the results of the spiked BCs precisely predicted the clinical trial results. Dr. Cherkaoui’s results establish that the performance of fully automated Rapid AST directly from positive blood culture bottles is consistently robust. The automation enhanced the percentage of readable inhibition zones and reduced the rate of isolates categorized with technical uncertainty. Summarizing, fully automated EUCAST RAST can substantially improve laboratory workflow by reducing hands-on time and removing the strong constraints linked to manual read-outs at precisely defined times.
Read the complete studies below: